
The O-C-O bond angle in CO 3 2- is 120 o the molecule is perfectly planar. Notice that the p x and p y orbitals are unaffected while the s and p z orbitals are been converted into two new degenerate orbitals of intermediate energy.Ĭonsider the carbonate ion.

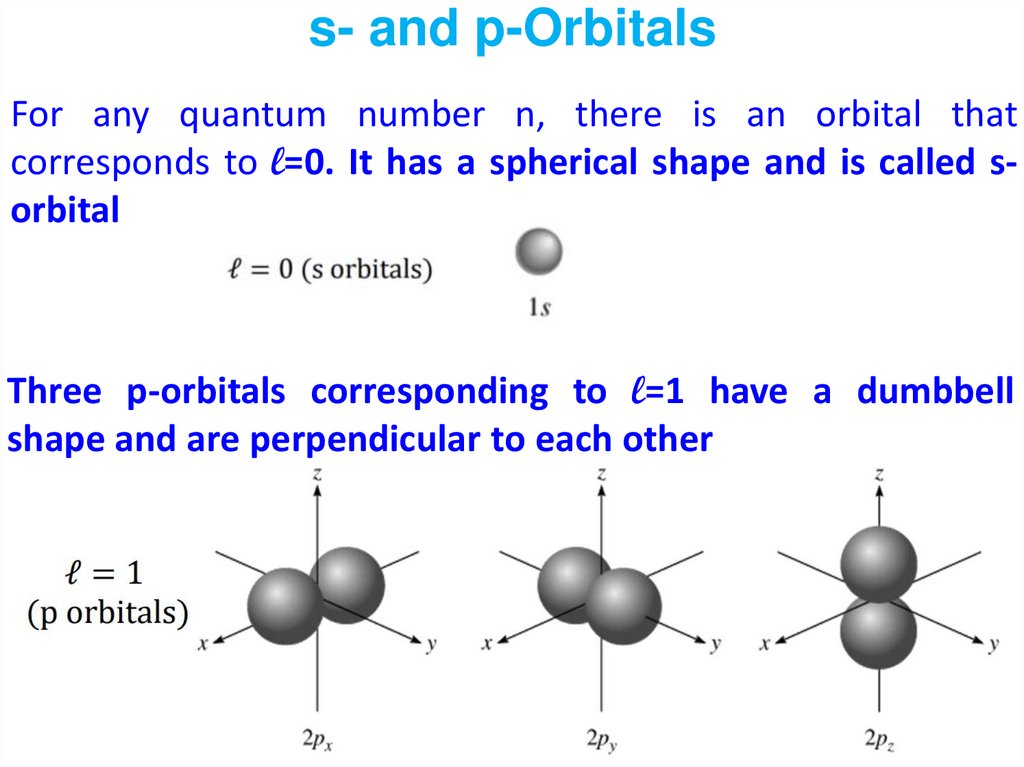
The energy diagram for the hybridization process is shown at the right. One sp hybrid orbital is oriented along the positive z axis the other is oriented in the opposite direction. Because two atomic orbitals were used to create the hybrid orbitals, two hybrid orbitals are formed. These two hybrid orbitals, the sp hybrid orbitals, are constructed from the carbon 2s and 2p z orbitals. One hybrid orbital provides direct overlap with an orbital on one oxygen atom the other hydrid orbital provides direct overlap with an orbital on the other oxygen atom. Valence Bond Theory requires two orbitals on the carbon atom that can be used to form sigma bonds with the two oxygens. The O-C-O bond angle in CO 2 is 180 o the molecule is perfectly linear. Basic atomic orbitals not employed in constructing the hybrid orbitals are unaffected by the hybridization process.Ĭonsider the carbon dioxide molecule. (The d orbitals may also be included, if necessary.) The hybrid orbitals have energies intermediate between those of the basic orbitals used to construct them. Valence Bond Theory manages this situation by creating hybrid orbitals that are linear combinations of the s, p x, p y, and p z orbitals in the valence shell. For an isolated carbon, the 2s, 2p x, 2p y, and 2p z wave functions are optimal, but in the presence of other atoms, where chemical bonding can occur, an alternate set of orbitals may be preferred. The carbon atom, in the example given above, will choose the set of wave function that minimizes its energy. Thus for n = 2, there are an infinite number of solutions, not just the 2s, 2p x, 2p y, and 2p z wave functions. Any linear combination of solutions to this equation is also a solution. Recall that the Schroedinger equation for the hydrogen atom is a linear differential equation. The solution to this problem is to create a new set of atomic orbitals. This geometry does not provide for effective overlap with the hydrogen atoms. The carbon 2p x, 2p y, and 2p z extend along the x, y, and z axes and as such form 90 o angles with each other. The carbon 2s orbital is spherical and as such extends toward all four hydrogen atoms. For example, in methane (CH 4) the carbon is at the center of the molecule and the hydrogens lie on the points of a tetrahedron. Second, to the extent that the orbitals have an orientation (the p x along the x axis, for example), the geometry is rarely consistent with the molecular geometry. First, these orbitals are not directed in a particular direction instead, they tend to spread out in all directions (or at least multiple directions). The basic s, p x, p y, and p z orbitals are unsatisfactory for two reasons. Good orbital overlap requires that the atomic orbitals on each atom (those orbitals overlapping to form the bond) be oriented directly toward the other atom. For a sigma bond, the overlap is along the line directly between the two nuclei. In Valence Bond Theory, a chemical bond between two atoms is the result of direct overlap of two atomic orbitals (one on each atom). Bonding with carbon sp 3 orbitals is shown in Figure 3 for a molecule of methane.Visualization of Atomic Orbitals: Hybrid Orbitals Visualization of Atomic Orbitals Hybrid Orbitals The number of hybrid orbitals produced equals the number of orbitals that have combined. Hybrid orbitals are orbitals of equal energy produced by the combination of two or more orbitals on the same atom. The sp3 orbitals all have the same energy, which is greater than that of the 2s orbital but less than that of the 2p orbitals, as shown in figure 2. Superscript 3 indicates that three p orbitals were included in the hybridization. To achieve four equivalent bonds, carbon’s 2s and three 2p orbitals hybridize to form four new, identical orbitals called sp 3 orbitals. Note that the 2s orbital and the 2p orbitals have different shapes. Two of carbon’s valence electrons occupy the 2s orbital, and two occupy the 2p orbitals. We know from experiments that a methane molecule has tetrahedral geometry. Figure 2: Carbon’s orbitals before and after sp 3 Hybridization How does carbon form four equivalent, tetrahedrally arranged covalent bonds by orbital overlap with four other atoms?


 0 kommentar(er)
0 kommentar(er)
